

Katie Aspray, Canterbury Christ Church University, Canterbury CT1 1QU. k_as...@hotmail.co.uk
The relationship between organisms and habitat structure has been the focus of investigation in many areas of ecology2,7. The influence of habitat structure on gastropods is a particularly popular field of research. This may be due to the close association gastropods have with surfaces1 Research has shown different relationships between habitat structure and gastropod abundance11, distribution5,2 species richness6 and growth9.
Here, I briefly review some of the relevant literature. The definition,
terminology and measurement of habitat structure can be difficult3,1,2.
For example, the terms habitat complexity8,
topographic complexity4 or patches5
have all been used as synonyms of habitat structure. This variability
of terminology creates difficulties in the comparison of results, derivation
of hypotheses and analysis of trends in the subject 2.
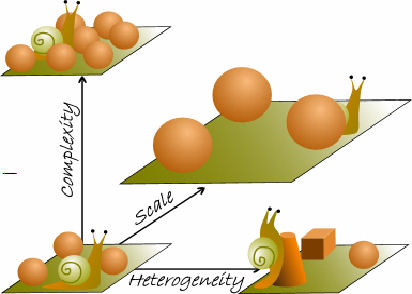
McCoy & Bell (1991) attempted to create a universal definition for habitat
structure as the "arrangement of objects in space"7.
This definition included a graphical model, with three major variables-
complexity, heterogeneity and scale (see figure).
'Complexity' refers to the amount of structure or variation attributable
to absolute abundance of individual structural components7,2.
'Heterogeneity' represents the kinds of structure or variation
attributable to the relative abundance of different structural components7,2.
'Scale' emphasises that the first two components must be commensurate
with the dimensions of the organisms being studied.
McCoy & Bell aimed to standardise the definition so that hypotheses could be generated and compared. Such comparisons would be achieved through standard definitions and terminology but also by appropriate measurement of habitat10, 1, 2.
Methods of measuring habitat structure vary greatly2,7. This may be due to the different aims of the research, differences in species and habitats studied, and the changeable nature of the environment7. Manipulation of habitat structure can often confound results2. Thus changing complexity through altering structural components can make it difficult to ascertain whether the response of gastropods is to heterogeneity or complexity of the habitat7,2. For example, the inclusion of two types of vegetation to increase complexity could mean response of an organism is to either an increase in complexity or an increase in components2. To overcome this, habitat indices can be used to measure changes in structure, thereby creating comparable measurements1. Some indices are based on specific structural components and are, therefore, limited in their use2. Others can be used to elucidate situations where habitat structure might affect gastropods1. Beck examined a variety of complexity indices to ascertain if these were relevant to gastropod biology1. Four indices were examined:
the fractal dimension (D),
vector dispersion (VD),
consecutive substratum height difference (Sdh2) and;
chain-and-type (chain).
Initially, Beck found that D was often correlated with gastropod density. However, in later research Beck found that when differences in complexity are small enough to create separation among the four indices, the difference in complexity was not great enough to affect gastropod ecology2. Therefore, when differences in complexity were great enough to have a significant affect on gastropod density, D was not definitively more significant than other indices. Sanson, Stolk & Downes (1995) also criticised the measurement of D, observing that it lacked consistency between spatial scales10. Beck concluded that it was unlikely that any single index would be suitable for every habitat and the use of several indices might give the most reliable measurements whilst still producing comparable results2.
Habitat structure has a strong influence on the local distribution of gastropods 2. In particular, there is a positive correlation between measurements of habitat structure and the density and abundance of gastropods4,1,2. Most studies found that in general, complex habitats had the highest density and abundance of gastropods, with some species-specific exceptions. For example, complexity had an effect on the density of two species of Australian marine gastropods (Austrocochlea porcata and Bembicium nanum) but not the variegated limpet Cellana tramoserica. C. tramoserica may not be affected by complexity because limpets usually occur clamped to plain surfaces where they can avoid desiccation 2. Ray and Stoner (1995) and Stoner, Lin & Hanisak (1995) also found a correlation between habitat structure and gastropod density, with a preference for increased seagrass biomass and density by the Queen Conch, Strombus gigas9,11. Ray and Stoner (1995) found juvenile conch over one year were found primarily in medium density seagrass, where there are lower rates of predation. Younger conch, found on bare sand, could be supported nutritionally but predation was a more important factor than food limitations9. Beck (2000) also suggested that protection from predators might be a function of habitat complexity 2.
By contrast, Beck (1998) suggested that complexity served primarily as protection from abiotic factors, such as wave surge and desiccation. Beck found that habitat structure affected gastropod species differently in varying habitats; gastropods responses were consistent with differences within the physical environment. For example, the density of gastropod species B. nanum, C. tramoserica and A. porcata were affected (varyingly) by habitat complexity in rocky intertidal habitats1.. However, Beck found these species were not significantly affected by changes in structural complexity in mangrove shores, where physical factors are less extreme than on rocky shores. This observation was supported by a later study by Ray-Culp, Davis, and Stoner8, which showed that predation on gastropods occurred independently of the degree of seagrass structure, suggesting that the higher numbers of gastropods in higher density seagrass was due to protection from physical factors.
Although a correlation was found between habitat structure and density of gastropods, Beck (1998) found no correlation between habitat structure and species richness. These results differ from those of Costil & Clement (1995), where an increase in habitat structure, in the form of plant communities, was correlated with an increase in gastropod species richness. These two studies differed greatly in the way habitat was measured, with Beck (1998) measuring habitat complexity as topographic changes and Costil & Clement (1995) measuring number of plant communities. This may explain the differing results and signify the importance of types of structural elements in the measurement of habitat1,2,3.
Habitat structure has also been found to influence dispersal patterns of gastropods12, 5. Underwood & Chapman found Littorina unifasciata showed different movements and dispersal in habitats of different complexity, moving faster and more directly in simple habitats. They later compared the movements of L. unifasciata with those of Nodilittorina pyramidalis4. This study showed that whilst both species prefered topographically complex habitats in terms of patterns of movement and abundance, L. unifasciata could be found on topographically simple habitats whilst N. pyramidalis actively avoided these areas. These studies and others have shown that patterns of movements by gastropods are influenced by complexity of habitat as well as being a function of other characteristics of the species12,4,5. It thus appears that habitat factors can affect various aspects of gastropod ecology. However, whilst there may be general relationships between habitats and gastropods, many of the relationships are influenced by the type of habitat and the characteristics of the species themselves 1,2,5. Knowledge of the relationships between habitat and gastropods could be important for gaining a proper understanding of issues such as distribution patterns, movements, habitat preferences and population dynamics.
References: