

Hemopoiesis in the freshwater architaenioglossan gastropod Pomacea canaliculata
J. A. CUETO
Laboratorio de Fisiología (IHEM-CONICET), Facultad de Ciencias
Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina.
E-mail: cuet...@fcm.uncu.edu.ar
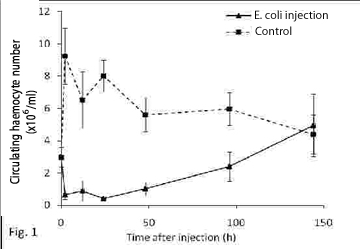
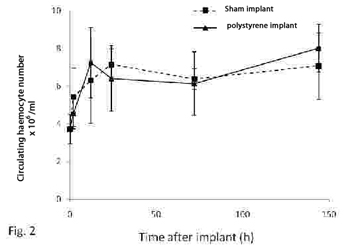
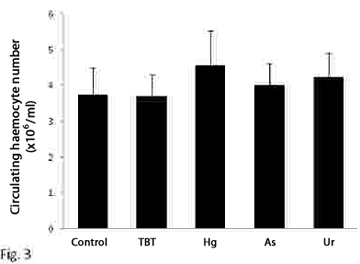
Hematopoiesis refers to the formation and development of mature blood cells involving proliferation, commitment and differentiation from early progenitors (stem cells) (Barreda and Belosevic, 2001). In vertebrates, the proliferative activity of bone marrow (the main hematopoietic organ), may reflect conditions that are associated with perturbations at the level of circulating cells. This organ is an important potential target for compounds affecting either the circulating mass of blood cells or the production of them (Lund, 2000; Travlos, 2006). In gastropods, the hemocyte production sites have been studied in only a limited number of pulmonate snails (van der Knaap el al., 1992; Sminia et al., 1981; Pan et al., 1958), while information on representatives of other gastropod taxa is either fragmentary or completely absent. Pomacea canaliculata is a freshwater snail whose natural range encompasses the lower Amazon and Plata basins, but which has been introduced in several South East Asian countries in the 1980s, where it became a serious agricultural pest (Halwart et al., 1994). With the aim of disclosing the site of haemopoiesis in P. canaliculata, we studied the behaviour of both circulating and tissue haemocytes in snails exposed to either immunological challenges or environmental pollutants like tributyltin (TBT), arsenate, mercury (Hg2+) and uranyle. The measured variables were the circulating haemocyte concentration and the immunohistochemical expression of a cell proliferation marker (“proliferating cell nuclear antigen”, PCNA, a cyclin expressed during the late G1 and S phases of the cell cycle), in (1) the circulation and the interstitial tissue of the posterior kidney, and (2) the capsule that may be formed in the foot muscle around an inserted foreign body. The posterior kidney was of special interest since large collections of haemocytes occur in the interstitial spaces of this organ and therefore was a likely place for haemopoiesis. Haemocyte ultrastructure of both the renal interstitium and the reactive capsule around a foreign body were also investigated with transmission electron microscopy. For the immunological challenges, a suspension of heat killed Echerichia coli was injected in the snail´s foot and a small polysterene ball was implanted also in the foot. Haemolymph samples were collected by puncturing the heart ventricle at different periods after the challenge. Bacterial injection resulted in a rapid decrease in circulating haemocyte counts, while full recovery only occurred only after several days (Fig. 1). Snails injected with apyrogenic water were used as controls. Fig. 2 shows that the circulating haemocyte concentration occurred in both sham-implanted and in polysterene-implanted snails. To assess the effect of environmental pollutants snails were exposed to TBT (60 ng/L) for 30 days and to sodium arsenate (As, 10 µg/L), mercuric chloride (Hg, 2 µg/L) and uranyle acetate (U, 30 µg/L) for 50 days in fully reconstituted water at 23-25°C. The circulating haemocyte count was not significantly affected by the environmental pollutants (Fig. 3). The ultrastructural study of the renal insterstitium in these snails, as well as that of PCNA expression are still in progress. |
 |
 |
|
 |
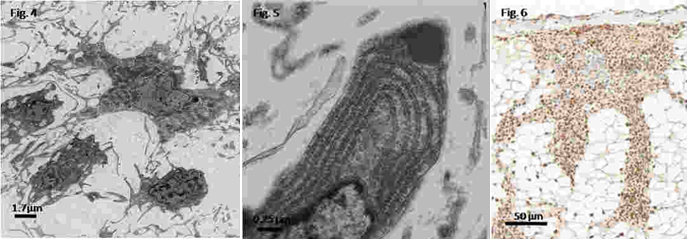
Electron microscopy examination of renal tissue haemocytes revealed the occurrence of hyalinocytes and only a few granulocytes. The surface of adjacent cells showed profuse interdigitation of filopodia (Fig. 4). Most these haemocytes showed an extensive cytoplasmic network of tubules and vesicles and numerous vacuoles containing low density material, numerous mitochondria, a well-developed rough endoplasmic reticulum (RER) as well as prominent Golgi profiles (Fig. 5). Renal interstitial haemocytes were vigorously proliferating 2 hours after bacterial injection, as indicated by PCNA marking of most cells (Fig. 6). Proliferating haemocytes were identified also within the vascular spaces of stimulated snails, while haemocytes of non-stimulated snails were mostly negative.
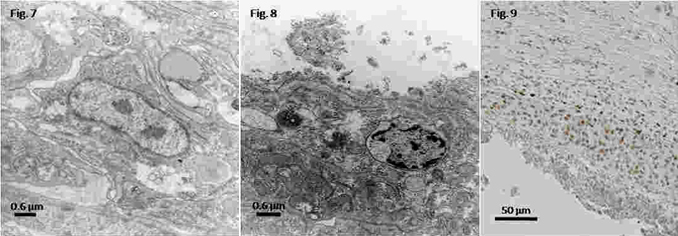
The reactive capsule around the polysterene implant was formed by at least two different layers: a compact inner one surrounding the implanted material and an outer layer of looser cells. The inner layer was mostly composed of cell debris and of degenerating haemocytes (Fig. 7), showing condensed nuclei and swollen mitochondria. Instead, the outer layer showed apparently healthy haemocytes, with relatively large nuclei and prominent nucleoli, suggesting an active state (Fig. 8). Also, some mitochondria and RER, as well as numerous free ribosomes, and many vesicles and tubules containing a material of varying electron density were observed. Hyalinocytes were also the most frequent haemocyte type found in capsules. PCNA positive nuclei occurred in the capsule outer layer, but not in the inner one (Fig. 9).
 |
 |
We interpret that
the rapid decrease in circulating haemocyte count after bacterial
challenge is caused by haemocyte involvement in bacterial trapping,
and by the subsequent formation of haemocyte clumps, which become
trapped and piled up in small vascular spaces, particularly those
of the renal insterstitium. Indeed, the posterior kidney in P.
canaliculata receives all the vascular efflux from the foot (Andrews,
1965). The subsequent reduction in circulating haemocyte number is
finally overcome by haemocyte reproduction which apparently also occurs
in the circulation. The continuous increase in circulating haemocytes
observed in both polystyrene-implanted and sham-operated snails may
be the result of haemocyte proliferation around the healing wound.
Therefore, we tentatively conclude that haemopoiesis may be triggered in P. canaliculata both by bacteria and by tissue injury, and that haemocyte proliferation may occur in the circulation, around wounds and foreign particles, and notably in tissues (as the renal interstitium) where haemocytes may become trapped. Except for the latter finding, and contrariwise to what we had initially hypothesised, we found no compelling evidence for a central haemopoietic role of the posterior kidney in P. canaliculata (as that of the “amebocyte producing organ” of some pulmonate snails (Sullivan & Spence 1994, Sullivan et al. 2004).
Acknowledgement
I would like to thank the Malacological Society of London for partly
supporting this research. Hugo H. Ortega and his lab have been extremely
helpful. I also wish to thank Alfredo Castro-Vazquez, Israel A. Vega
and Maximiliano Giraud-Billoud.
References
ANDREWS, E. B. 1965. Journal of Zoology, 146: 70-94.
BARREDA, D. R. and BELOSEVIC, M. 2001. Dev Comp Immunol, 25(8-9):
763-89.
LUND, J.E. 2000. In: Schalm's Veterinary Hematology, 44-48.
TRAVLOS, G. S. 2006. Toxicologic Pathology, 34:548–565.
PAN, C. T. 1958. Bull Mus Comp Zool, 119: 237-99.
SMINIA, T. 1981. In “Invertebrate Blood Cells”. Vol. 2,
p. 191-232.
VAN DER KNAAP, W. P.; ADEMA, C. M.; SMINIA, T. 1992. Comparative Haematology
International, 3(1): 20 - 26
HALWART, M. 1994. International Journal of Pest Management, 40: 199-206.
SULLIVAN, J.T. and SPENCE, J.V. 1994. Journal of Parasitology, 80:
449-453.
SULLIVAN, J.T.; PIKIOS, S.S.; ALONZO, A.Q. 2004. Journal of Parasitology,
90: 92-96.