

Are morphological and molecular perspectives of anomalodesmatan phylogeny reconcilable?
André F. Sartori
Department of Earth Sciences, University of Cambridge, Downing Street,
Cambridge, CB2 3EQ, UK, afs...@cam.ac.uk
Until recently considered a subclass of its own, anomalodesmatan bivalves are, at first sight, a heterogeneous assemblage. The group comprises shallow and deep burrowing forms, epifaunal species that attach to hard substrata by either byssal threads or cementation, and has also evolved life habits rarely found in bivalves, such as carnivory and tube-dwelling. Despite the difficulties posed by the disparate morphologies that attend this plethora of ecological strategies, the classification of extant anomalodesmatans has been relatively stable since the Treatise on Invertebrate Paleontology in which Newell (1969) divided the group into the superfamilies Pholadomyoidea, Pandoroidea, Poromyoidea and Clavagelloidea. With the exception of a split of the Pandoroidea into two superfamilies (Thracioidea and Pandoroidea), proposed by Boss (1978), supported by Yonge & Morton (1980) and formalised by Morton (1982), most suggested changes to Newell's scheme were either contested or ignored by subsequent authors.
When the first computer-driven cladistic analysis of extant anomalodesmatans
(Harper et al., 2000) corroborated the division of taxa into those
five widely accepted groups (Fig. 1, morphology), it seemed that
Newell's classification with the modification introduced by Boss
(1978) reflected well the phylogeny of the group. However, as soon
as molecular studies of bivalves were intensified, the resulting
cladograms implied a radically different picture (Dreyer et
al., 2003; Harper et al., 2006). None of the traditionally
accepted superfamilies was rendered unambiguously monophyletic.
Instead, the molecular trees showed two branches blending members
of the Thracioidea, Pandoroidea and Clavagelloidea (thraciid and
lyonsiid clades), while representatives of the Poromyoidea appeared
in a basal polytomy (Fig. 1, 18S rRNA).
Although thraciid and lyonsiid clades were resolved with moderate
to high branch support, morphological synapomorphies for these groups
could not be recognised, precluding the possibility of formulating
diagnoses and incorporating them into the classification of anomalodesmatans.
This could indicate that convergence has been so common in the group
as to mask all phylogenetic signal from anatomical traits. Alternatively,
the conflicting results may be due to homologies being misinterpreted
and insufficient knowledge of the variation within each superfamily.
If the latter is the case, reinterpreting homologies and increasing
the number of taxa and morphological characters sampled should resolve
the issue, revealing the apomorphies shared by each clade. Performing
such investigation is one of the objectives of my ongoing PhD.
Preliminary analysis
The preliminary analysis of my dataset presented during the 2007
World Congress of Malacology and published herein includes 20 taxa
in the ingroup and Cardita ventricosa as the outgroup.
Choice of the outgroup was based on the now accepted view that anomalodesmatans
are basal heterodont bivalves. Fifty morphological characters were
scored and treated as unordered and equally weighted. When available,
data was also obtained from the literature. Searches for the most
parsimonious tree(s) were performed in PAUP 4.0b10 using heuristic
methods. Percentage bootstrap figures were obtained to indicate
support for the branches.
Two most parsimonious cladograms of length 156 with a consistency
index of 0.47 and a retention index of 0.63 were found. The strict
consensus tree (Fig. 2) recovers both the thraciid and lyonsiid
clades although only the former is resolved with moderate branch
support. Characters supporting these clades and good candidates
for apomorphies of the groups are, for the thraciid clade: pallial
glands, which are located on the right mantle lobe of all representatives
of the group (character 1; Fig. 3A); reduction of the byssal apparatus,
with the byssal groove barely visible at the heel of the foot (character
2; Fig. 3B) and; loss of the arenophilic glands, organs responsible
for the attachment of sediment to the periostracum of most anomalodesmatans
(Sartori et al., 2006). The glands are absent in all members of
the thraciid clade but the periplomatids Offadesma angasai
and Cochlodesma praetenue (character 3; Fig. 3C). For the
lyonsiid clade, the apomorphies are: abundant siphonal tentacles
organized in more than one concentric row and with a ciliated receptor
at the distal end (character 4; Fig. 3D) and; presence of modified
apical filaments at the crest of each ctenidial plica (character
5; Fig. 3E). Apical filaments also occur in Pholadomya candida
and Offadesma angasai.
Other interesting results that have not yet been tested by molecular
studies are the non-monophyly of the Pholadomyoidea and the position
of the Periplomatidae. Pholadomyoidea is rendered paraphyletic in
the analysis because the genus Parilimya cluster with the
remaining anomalodesmatans instead of with Pholadomya.
This is supported by the complete reduction of the heterodont dentition
in Parilimya and the remaining anomalodesmatans (character
6; Fig. 3F). The external, parivincular ligament which has been
traditionally used to justify grouping Parilimya with Pholadomya
is, in fact, plesiomorphic. The family Periplomatidae, represented
in the analysis by O. angasai and C. praetenue,
appears in a surprising position within the thraciid clade. Periplomatids
have long been considered closely related to laternulids, with whom
they share a radial crack through the umbonal region of the shell
and chondrophores supported by a buttress (character 7; Fig. 3G).
We must assume these to be convergent structures if we accept the
topology of the strict consensus tree.
Summing up, it is encouraging to see some accordance arising between
the morphological and molecular datasets. However, the results showed
herein are preliminary and liable to change as the investigation
progresses, in particular because homoplasy levels are high, as
indicated by a low CI, and few groups show medium to high bootstrap
support. Work in progress includes adding members of the Poromyoidea
to the morphological survey and performing a combined morphological
and molecular analysis.
Literature Cited
| Boss, K.J. 1978. Taxonomic concepts and superfluity in bivalve nomenclature. Philosophical | |
| Transactions of the Royal Society of London B284: 417–424. | |
| Dreyer, H., G. Steiner and E. M. Harper. 2003. Molecular phylogeny of Anomalodesmata (Mollusca: | |
| Bivalvia) inferred from 18S rRNA sequences. Zoological Journal of the Linnean Society 139: 229–246. | |
| Harper, E.M., E.A. Hide and B. Morton. 2000. Relationships between the extant Anomalodesmata: | |
| a cladistic test. In: Harper, E.M., J.D. Taylor and J.A. Crame, eds. The Evolutionary Biology of the Bivalvia. Geological Society of London Special Publications 77: 129–143. | |
| Harper, E.M., H. Dreyer and G. Steiner. 2006. Reconstructing the Anomalodesmata (Mollusca:Bivalvia): | |
| morphology and molecules. Zoological Journal of the Linnean Society 148: 395–420. | |
| Morton, B. 1982. The functional morphology of Parilimya fragilis (Bivalvia: Parilimyidae nov.fam.) | |
| with a discussion on the origin and evolution of the carnivorous septibranchs and a reclassification of the Anomalodesmata. Transactions of the Zoological Society of London 36: 153–216. | |
| Newell, N.D. 1969. Classification of Bivalvia. In: Moore, R.C., ed. Treatise on Invertebrate | |
| Paleontology. Part N, Vol. 1 Mollusca 6, Bivalvia. Boulder Colorado: Geological Society of America and Lawrence, Kansas: University of Kansas Press, N205–N224. | |
| Sartori, A.F., F.D. Passos and O. Domaneschi. 2006. Arenophilic mantle glands in the Laternulidae | |
| (Bivalvia: Anomalodesmata) and their evolutionary significance. Acta Zoologica (Stockholm) 87:265–272 | |
| Yonge, C.M. and B. Morton. 1980. Ligament and lithodesma in the Pandoracea and Poromyacea with | |
| a discussion on the evolutionary history of the Anomalodesmata (Mollusca: Bivalvia). Journal of Zoology 191: 263–292. | |
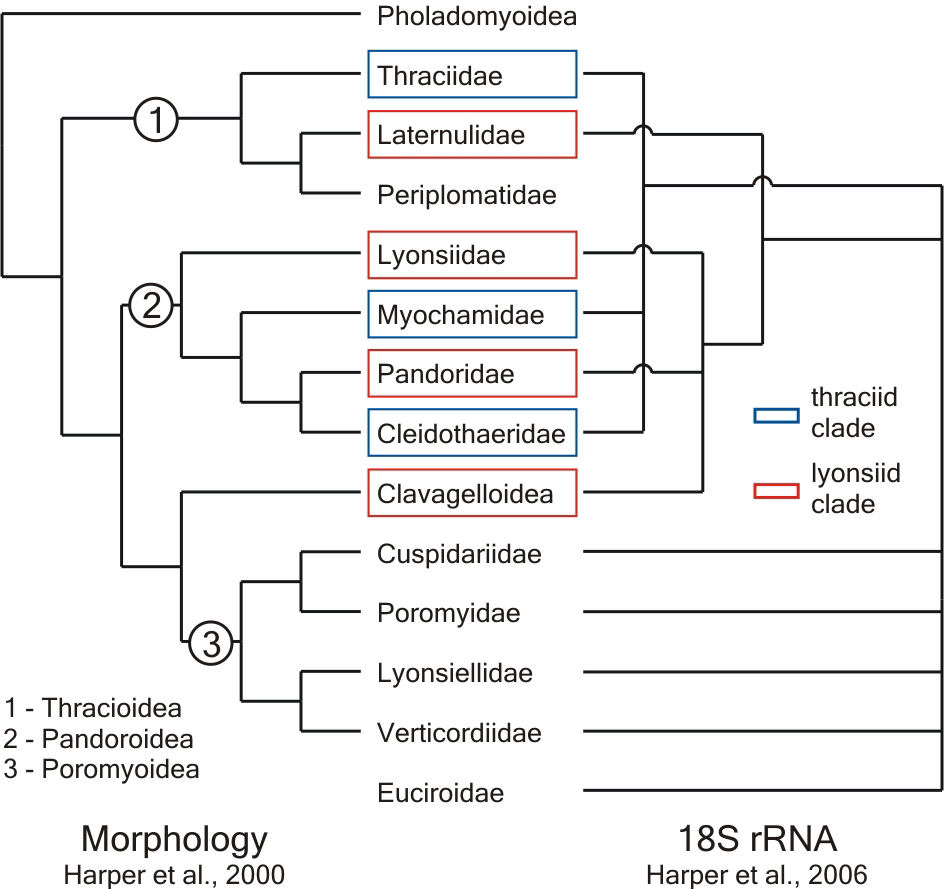 |
Fig. 1. Comparison of the most recently published cladograms of the Anomalodesmata, based on morphological and molecular data. |
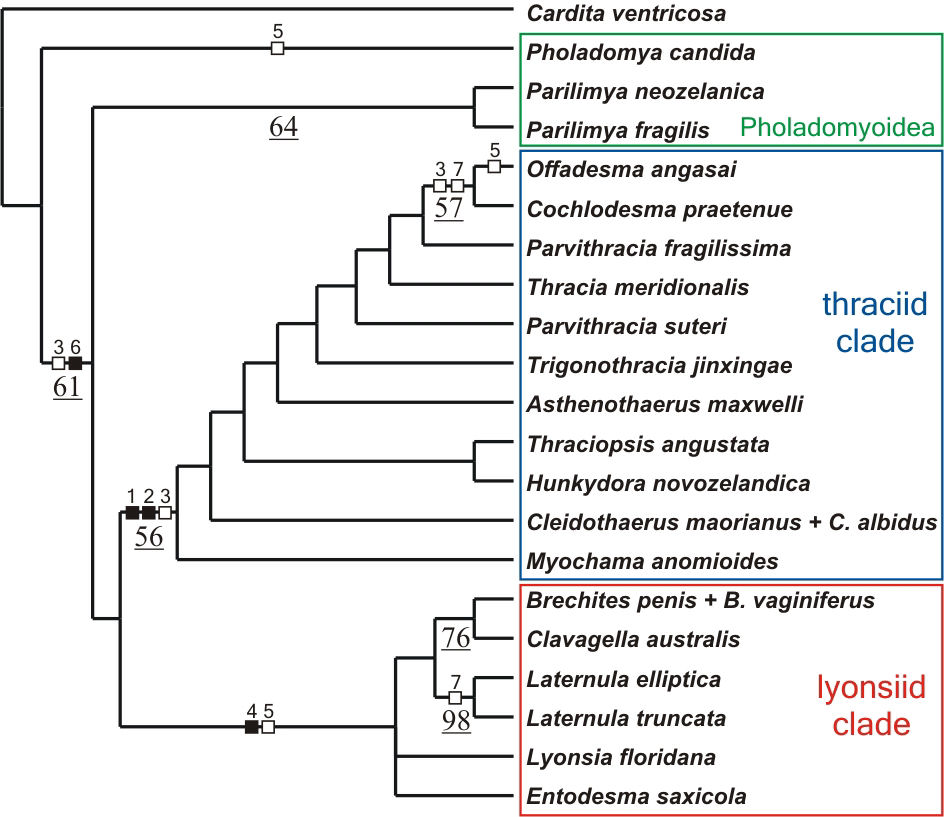 |
Fig. 2. Strict consensus tree of two most parsimonious cladograms illustrating the relationships of extant anomalodesmatan as inferred from morphological data. Bootstrap values >50% are given below branches and underlined. Filled squares indicate non-homoplastic character state transitions, open squares indicate convergent changes or reversals [homoplastic changes]. Numbers above squares denote the characters as numbered in the text. |
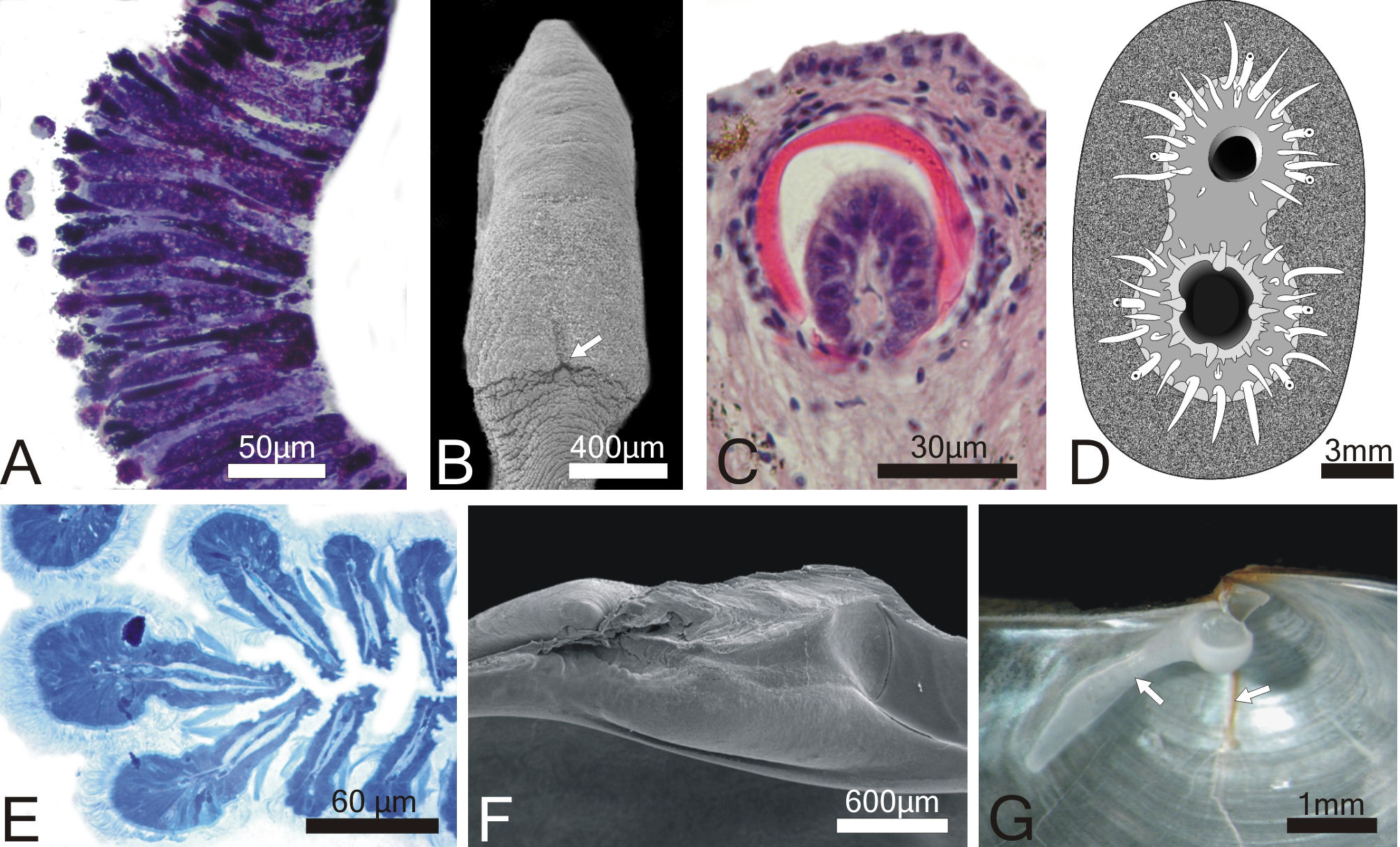 |
| Fig. 3. Morphological
characters plotted on the strict consensus tree. A. Pallial glands (character 1), transversal section through the right mantle lobe of Thracia meridionalis; B. Reduced byssal groove (char. 2), scanning electron micrograph of the sole of the foot of T. meridionalis; C. Arenophilic glands (char. 3), transversal section through the siphons of Laternula boschasina; D. Abundant siphonal tentacles in several successive concentric rows (char. 4), posterior view of the siphons of L. elliptica; E. Enlarged apical filaments (char. 5), transverse section through the ctenidial filaments of L. elliptica; F. Complete reduction of the heterodont dentition (char. 6), scanning electron micrograph of the hinge of Parilimya neozelanica; G. radial crack and buttress (char. 7), ventro-lateral view of the hinge of Periploma compressum. |