

Mating behaviour of Deroceras
Heike Reise,
State Museum of Natural History, Görlitz, Germany
Our grant application concerned the recording of mating behaviour
in a number of Deroceras slug species; particularly we proposed
to address two separate questions.
Question 1: Is extreme morphology associated with extreme
behaviour in Deroceras gorgonium?
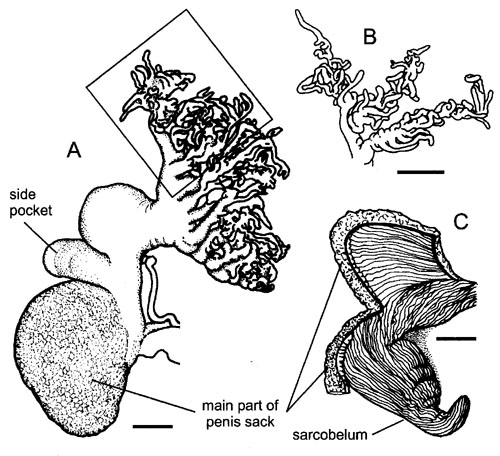
Figure 1: Morphology of distal male genitalia of Deroceras gorgonium. A. The whole penis. B. Component part of the penial gland (position shown boxed in A). C. The sarcobelum revealed by cutting open the main part of the penis sack. Scale bars = 1 mm. |
One striking aspect of mating in many Deroceras
species is the eversion of the appending penial gland over the
partner to transfer a secretion. The timing of the transfer
in some species, just after sperm exchange, rules out a function
such as synchronising the copulation; instead, it suggests a
role in sperm competition. The secretion may act as an anti-aphrodisiac
to hinder re-mating, or as an allohormone that manipulates the
partner into using the sperm just received, thus being comparable
to the function of the helicid love dart (Chase and Blanchard,
2006) We examined the mating behaviour of Deroceras gorgonium, the species with the largest and most branched penial gland (Fig. 1) amongst the several (more than 100) species of this genus. We collected specimens from a small area of endemism from Crete, and we video recorded 22 matings in our laboratory in Germany (Reise et al., in press). Additionally, we collected specimens of six other Deroceras species, of which we also studied the mating behaviour in the laboratory; however, this data have yet to be analysed. |
Our results show that in Deroceras gorgonium the penial gland
is everted either at or just after the very rapid (< 1s) sperm
exchange. The eversion looked like time-lapse recordings of growing
roots; often, the branches of the penial gland seemed to be clumsily
misdirected, initially sticking up in the air and consequently not
always covering the partner before being retracted. Often, the glands
were also everted under the partner's body; such function was not
recorded in the mating behaviour of other species we studied. We suggest
that this is the function for which the unusual size and shape of
the penial gland of Deroceras gorgonium are adapted.
 |
The mating behaviour of Deroceras gorgonium turned
out to be unusual in other respects as well. First,
the courtship process was at least 6, and sometimes over 9, hours
longer compared to other Deroceras species. Secondly,
it always consisted of many short bouts of different behaviour,
including periods of apparent inactivity. Thirdly,
much of the courtship consisted of stereotyped waving of sarcobela,
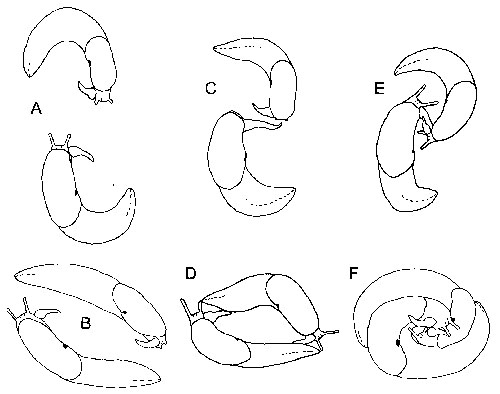
reminiscent of two circling swordsmen afraid to strike (Fig. 2). In other Deroceras the finger- or hand-like sarcobelum is stroked along the side of the partner's body. However, in Deroceras gorgonium the sarcobelum is unusually long and pointed and during the first hours of courtship it is merely waved in front of the partner's face; when it does touch the partner it takes hours before it does so with more than its tip. |
Figure 2: Courtship of Deroceras gorgonium. A and B. Distant waving and circling during early courtship. C and D. Closer waving and circling with body contact characteristic of later courtship. E. Close contact between sarcobela. F. Close yin-yang, shortly before sperm exchange.
This waving is regularly coordinated with the circular moves of the
partners that eventually lead to swapping positions (Fig. 2). It is
possible that these circular moves and action of the sarcobela serve
in assessing partner's size or sensing components of the partner's
mucus in order to receive information about its physiological state.
During our recordings we observed the transfer of drops of secretion,
thus confirming for the first time in Deroceras, such a function
for the sarcobelum.
Currently, we are conducting further experiments, regarding a more
accessible species, Deroceras panormitanum, to investigate
further the function of the appending penial gland.
Question 2: Assessing whether mating behaviour functions as
an isolation mechanism between sibling Deroceras species.
Although Deroceras is the most speciose genus of terrestrial
slugs there is very little variation in the external morphology of
different species. This could be the result of (i) sexual selection
that drives divergence of reproductive anatomy and mating behaviour
between allopatric populations, and (ii) selection against hybridisation
in the event the populations meet.
Previously, we have identified extremely narrow zones of contact between
pairs of sibling Deroceras species: between Deroceras
praecox and Deroceras rodnae in the Babia Gora mountains
of Poland, and between Deroceras praecox and Deroceras
fatrense in the Mala Fatra mountains of Slovakia. These pairs
of species are morphologically distinguishable only by differences
in the shape of their penis, and specifically the sarcobelum. Subtle
differences in the mating behaviour of the allopatric populations,
e.g. the way in which the sarcobelum strokes and the timing of the
different stages of mating, were recorded previously (Reise, 1995).
In the present study we examined the mating behaviour of pairs of
sibling species across these narrow contact zones. Our objective was
to assess whether mating behaviour gradually intergrades due to introgression,
or whether it is more distinct in the contact zone as a result of
selection against hybridisation.
In each geographical area we collected both sibling pecies from populations
whose habitats were well away from the zone of contact, and we compared
interspecific and intraspecific mating behaviour. We then repeated
this for populations whose habitats were only a few hundred metres
on either side of the zone of contact. Finally, we studied the mating
behaviour between individuals of both sibling species collected within
the zone of contact, in habitats where individuals of both species
are found together with the occasional apparent intermediate. All
recordings took place in our laboratory using specimens collected
from the wild within the previous few weeks.
So far, we have recorded 211 mating events. The analysis, though,
is still incomplete and we wish to remain blind to the pattern of
results until we have finished scoring. So far, we observed that interspecific
crosses, lead to mating behaviour, even when individuals come from
distant populations; however, many of these mating events fail to
lead to successful sperm exchange.
In the future, we plan to follow up these comparisons on anatomy and
mating behaviour with a genetic analysis of gene flow across Deroceras
sibling species inhabiting the contact zones.
The Malacological Society of London provided the funds to improve
our digital video system for analysing the mating behaviour of Deroceras
slugs, and we are extremely grateful for this financial assistance.
Literature cited
Chase, R. and K.C. Blanchard, 2006. The snail's love-dart delivers
mucus to increase paternity.Proc. R. Soc. Lond. B., 273,
1471-1475.
Reise, H., 1995. Mating behaviour of Deroceras rodnae Grossu
et Lupu, 1965 and D. praecox Wiktor, 1966 (Pulmonata: Agriolimacidae).
Journal of Molluscan Studies 61, 325-330.
Reise, H., S. Visser, and J.M.C. Hutchinson. In press. Mating behaviour
in the terrestrial slug Deroceras gorgonium: is extreme morphology
associated with extreme behaviour? Animal Biology
[Figs. 1 and 2
are reproduced from Animal Biology 57(2) with permission.]