

Steven Brady,
Yale School of Forestry and Environmental Studies, 370 Prospect Street, New Haven, CT, USA. stev...@yale.edu
Background
Modern landscapes are dominated by human use (Vitousek et al. 1997). The attendant network of roads across this landscape is pervasive. The presence and use of roads on the landscape contribute a suite of direct and indirect ecological effects, ranging from habitat conversion and fragmentation to vehicle-wildlife collisions to runoff induced contamination (Forman and Alexander 1998). In temperate climates, road deicing agents—especially road salt—are among the leading contaminants to accumulate in roadside environments.
The results of investigations of environmental influence from roads typically indicate negative consequences for a diverse suite of taxa. Most often, these outcomes are inferred from a strictly ecological perspective. While valuable, such an approach limits insight to immediate effects on demography, or proxies thereof. However, incorporation of evolutionary perspectives can extend insight into the long-term consequences of roads on wild populations. By recognizing that populations can differ genetically on very local scales—and therefore undergo rapid evolutionary responses to dynamic environments—we can design field and laboratory studies to effectively detect variation at biologically important scales in a way that will greatly advance our understanding of the fate of species in human modified landscapes (Western 2001).
Goals and objectives
The overarching goal of this research is to develop understanding of the response of wetland dwelling mollusks to human land development. In particular, I am investigating the influence of road adjacency on the planorbid, Planorbula armigera. Three objectives guide this investigation:
Objective 1 : Assess the distribution of P. armigera across a suite of roadside and woodland wetlands.
Objective 2 : Determine whether biologically relevant physical and chemical characteristics within host wetlands vary with respect to road adjacency.
Objective 3 : Evaluate whether local populations of P. armigera have undergone phenotypic divergence with respect to road adjacency.
Approach and study sites
I have begun fulfilling these objectives by employing a three-tiered approach that combines field surveys, water chemistry analyses, and laboratory experiments. Study sites of this research are comprised of temporary wetlands located in the Yale Myers Forest in northeastern Connecticut, USA.
Field surveys: P. armigera depend critically on temporary wetland habitat. Such temporary wetlands in this region tend to be small and shallow by comparison to permanent wetlands. As a result, road runoff contaminants can become highly concentrated. In an average year, these temporary wetlands fill with water in the fall, and remain saturated until mid- to late-summer, after which time complete drying of the wetland occurs.
In May 2009, I surveyed a suite of 28 randomly selected temporary wetlands located within Yale Myers Forest. Of these, 12 wetlands are located within 10 meters of a primary road (hereafter roadside wetlands) while 16 wetlands are located at a distance greater than 200 meters from any paved road (hereafter woodland wetlands). I recorded the following suite of physical and chemical parameters at each wetland: pH, dissolved oxygen, specific conductance, depth, and temperature. I used standard, timed dip net procedures to search each wetland for P. armigera.
Water chemistry analyses: In May 2009, I collected water samples from a subset of 10 wetlands (five roadside and five woodland). Water sample analyses were conducted at the University of Connecticut, Storrs, CT, USA. The following analytes were tested in each sample: Cl, NO3, PO4, SO4, total Hg, and total organic C.
 |
Laboratory experiments: In August 2008, I collected a target number of 30 individuals from each of eight wetlands—four roadside and four woodland—in which I detected P. armigera. All collected individuals were returned to Greeley Laboratory, New Haven, CT, USA, and reared in 12-liter glass aquaria grouped according to wetland origin. I have been rearing these populations through several generations of offspring. Upon reaching the 4th filial generation—so as to minimize non-genetic environmentally inherited effects—I will conduct an acute road salt exposure experiment on a subset of individuals from each population in order to identify a survival curve associated with varying concentrations of road salt. Following this experiment, I will begin a long-term artificial selection experiment to assess the capacity for evolution of P. armigera in response to road salt. |
| Fig. 1. Planorbula armigera. The lab-reared individual seen here belongs to the second filial generation descendent from a roadside wetland. |
Preliminary analyses and results
I used MANOVA and simple linear models to assess first whether any of the measured physical or chemical components varied with respect to wetland type (i.e. roadside versus woodland). I then used logistic regression to evaluate whether any of the variables predicted the presence of P. armigera across the suite of 28 wetlands.
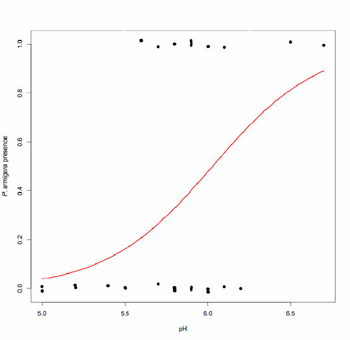 Results indicated that among the in situ wetland parameters, only specific conductance varied with respect to wetland type. On average, roadside wetlands had a specific conductance of 745 µS as compared to 25 µS in woodland wetlands. Similarly, three of the chemical analytes—Cl, total organic C, and total Hg—varied by wetland type (all p < 0.05). On average, Cl was detected at a level of 278 mg/l among roadside wetlands as compared to 1.2 mg/l among woodland wetlands. On the other hand, both total Hg and total organic C were higher in woodland wetlands (2.5 ng/l and 3.7 µg, respectively) compared to roadside wetlands (0.58 ng/l and 1.2 µg). Finally, among all of the wetland parameters, only pH predicted the distribution of P. armigera (df = 27, z = 1.97, p = 0.049, Fig. 2).
Results indicated that among the in situ wetland parameters, only specific conductance varied with respect to wetland type. On average, roadside wetlands had a specific conductance of 745 µS as compared to 25 µS in woodland wetlands. Similarly, three of the chemical analytes—Cl, total organic C, and total Hg—varied by wetland type (all p < 0.05). On average, Cl was detected at a level of 278 mg/l among roadside wetlands as compared to 1.2 mg/l among woodland wetlands. On the other hand, both total Hg and total organic C were higher in woodland wetlands (2.5 ng/l and 3.7 µg, respectively) compared to roadside wetlands (0.58 ng/l and 1.2 µg). Finally, among all of the wetland parameters, only pH predicted the distribution of P. armigera (df = 27, z = 1.97, p = 0.049, Fig. 2).
Since collecting eight populations of P. armigera one year ago, two generations of offspring have successfully reproduced in six of the eight populations, while two of the populations were unsuccessful in the laboratory. Following the successful production of one more generation of offspring in each population, I will begin conducting the acute exposure and artificial selection experiments.
Fig. 2 Planorbula armigera presence-absence with respect to pH. Observed presence-absence is plotted (closed circles) for each wetland (n=28). Overlapping values have been jittered so all data points are visible. Predicted values based on logistic regression are indicated by the fitted red line.
Discussion
In the suite of wetlands surveyed here, P. armigera was unconditionally absent at pH values below 5.6, suggesting this may be an important parameter governing the distribution of this species.
That Cl was detected at such high concentrations suggests that roadside wetlands are receiving high rates of road salt run off. That total Hg and total organic C were lower in roadside wetlands suggests that there may be an interaction between road adjacency and the chemical cycling process. Together, these results suggest that roadside and woodland wetlands are 
very different environments. Thus, it surprising that the distribution of P. armigera was not influenced by road adjacency. This suggests either that P. armigera is insensitive to the contaminants associated with roadside environments, or that local populations have evolved tolerance to these environments. These alternative outcomes will begin to be resolved following insight gained from the upcoming acute exposure and artificial selection experiments. Specifically, I will test the tolerance of each population to road salt to evaluate the hypothesis that local populations have evolved increased tolerance to road salt.
Acknowledgements
I thank D. Skelly, M. Urban, P. Turner, and S. Alonzo for helpful conversations in the development of this project. J. Bushey provided key assistance in water chemistry sampling and analyses. A. Brady, S. Bolden, and J. Burmeister all provided valuable field and laboratory assistance. I am grateful to the Malacological Society of London and the Carpenter-Sperry-Mellon Small Grant Program ( Yale University) for supporting of this research.
Literature Cited
Forman, R. T. T. and L. E. Alexander. 1998. Roads and their major ecological effects. Annual Review of Ecology and Systematics:207-231.
Vitousek, P. M., H. A. Mooney, J. Lubchenco, and J. M. Melillo. 1997. Human domination of Earth's ecosystems. Science 277:494-499.
Western, D. 2001. Human-modified ecosystems and future evolution. Proceedings of the National Academy of Sciences of the United States of America 98:5458-5465.