Report on a Centenary
Research Grant for 2003
Ontogeny of Mantle Cilia in the Bivalve Larvae of
Crassostrea gigas and Ostrea edulis
SAMUEL STANTON, Institute of Marine Sciences, School of Biological
Sciences, University of Portsmouth, Ferry Road, Portsmouth, PO4 9LY,
UK sam....@port.ac.uk
The anatomy of bivalve veligers is much less understood than that of
the adults. The study of bivalve larval anatomy and its ontogeny brings
challenges of methodology, requiring the modification of electron microscopy
techniques. This study used critical point drying techniques to investigate
mantle ciliation, because evaporation drying via HMDS leaves mucus deposits
which obscure the cilia. To investigate the mantle folds and their associated
cilia patterns, larvae were cracked open using fine glass needles. Some
larvae were also decalcified in ascorbic acid.
I am currently studying Crassostrea gigas and Ostrea edulis,
although ultimately a number of other species of economic significance
such as teredinids and pectinids will be included to provide a comparative
account of the development of these structures. At present there is
no comprehensive review, with its inherent consistency of method, covering
several species.
 |
Previous
studies have identified complex, organised and varied groupings
of cilia on the mantle of Pecten maximus larvae, with five
distinct cilia types, each specific to regions of the mantle (Cragg,
1991). The present study found a similar organization in C.
gigas larvae, and has followed the modification of this ciliation
through development. The mantle rim of veliger stage larvae has
few cilia; but the ciliation can be mapped according to the different
cilia groupings. Along the mantle rim a bud structure (fig.
1), probably the developing gill bud, appears to be the starting
point of the cilia tracts on the inner mantle rim.
|
| Fig.
1. Cilia on the developing gill bud and beginnings of the twin tract
on the inner mantle rim. |
|
 |
Originating
on the gill bud/mantle junction, cilia appear in small groups of
approximately 5 or 6, but without obvious organisation. From this
point a twin tract of cilia spreads along the anterior mantle rim,
steadily changing to a single tract, before finally becoming a less
coherent scattering of cilia (fig. 3a).
Changes in the overall pattern throughout larval development have
begun to be mapped as shown in fig. 3.The
mantle ciliation increases in both density and complexity through
the pediveliger stage (see fig. 3b).
The twin tract of cilia extends around the ventral region forming
a dense area of ciliation before becoming less distinct towards
the anterior. |
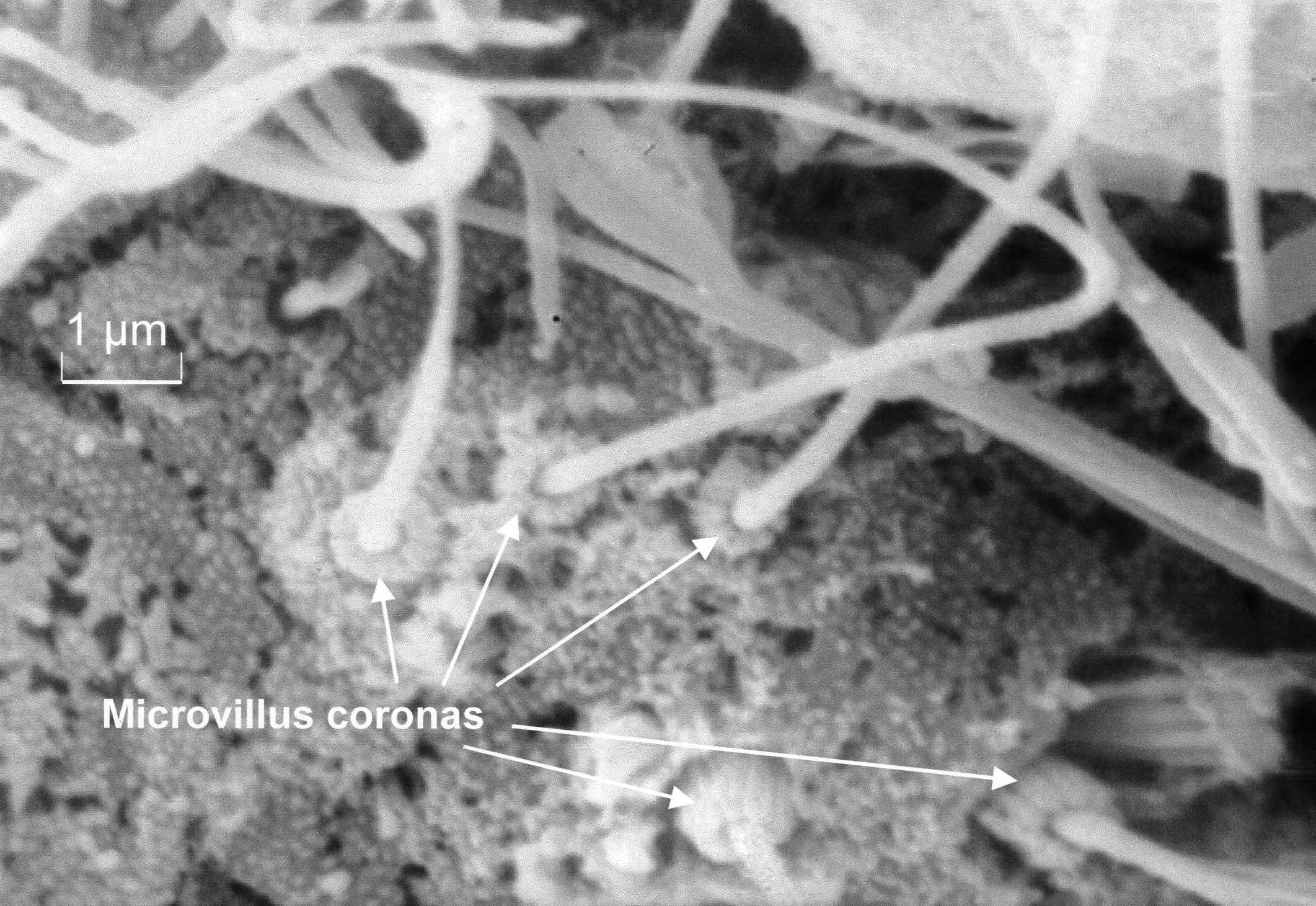
| Fig.
2. Microvillus bases on cilia of posterodorsal notch. |
|
These cilia have
been observed beating in live larvae, especially in moribund larvae
where they are not obscured by the preoral velum cilia. This beating
suggests a distinct function, possibly mantle cavity aeration or interaction
with velum cilia. The region of the gill bud, a starting point for this
cilia development in the veliger, becomes densely ciliated; and the
gill itself also features rows of cilia along the margins of the folds.
In both the veliger and pediveliger stage, ciliation in the region of
the anus is comparable to the anal grouping previously identified in
O. edulis by Waller (1981). This postanal
tuft includes an interesting cilia group with specialised cilia bearing
a corona of approximately nine microvilli around the base of each cilium
(fig. 2), suggesting a mechanoreceptive
function.
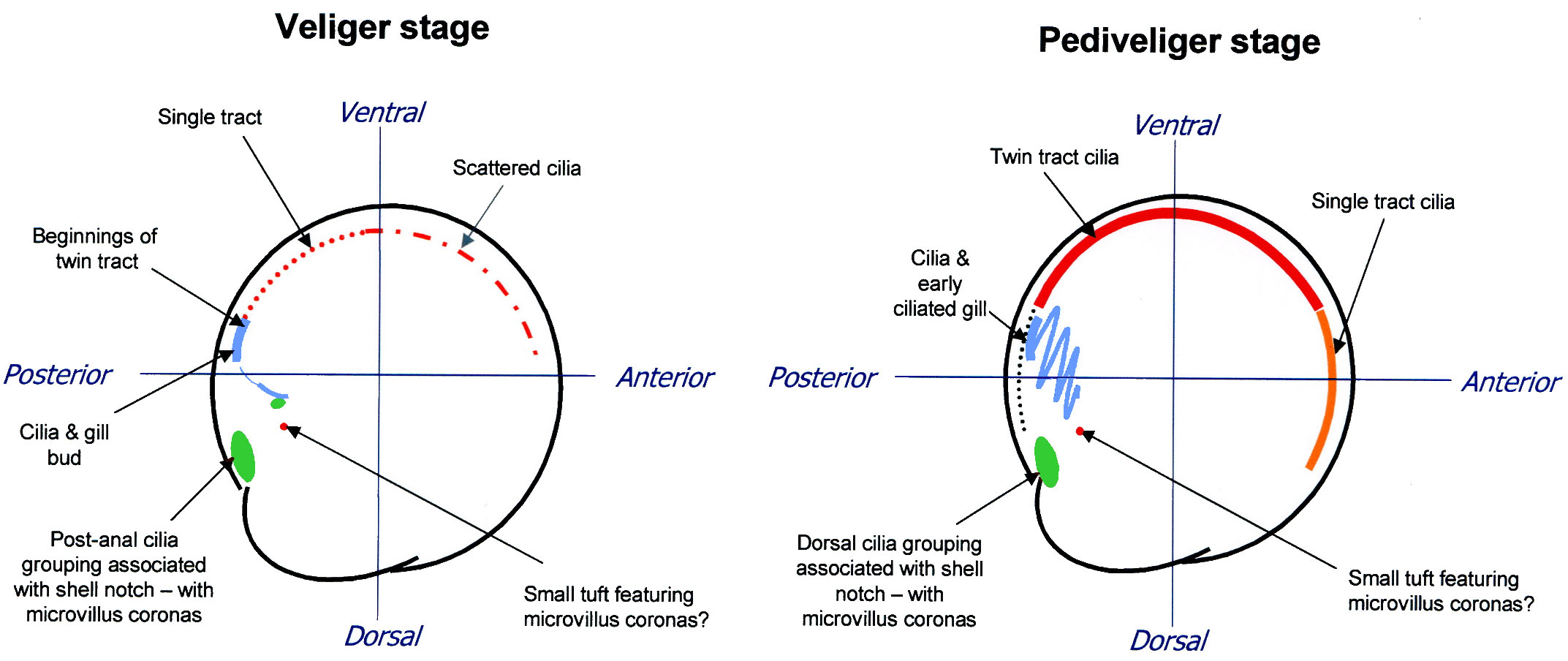 |
| Fig. 3. Preliminary
ciliation maps for (a) veliger and (b) pediveliger |
Waller (1981) proposed
that the beating of this cilia group assists excrement evacuation; however
this function fails to account for the association of this group with
the posterodorsal notch in the shell. This shell feature, just posterior
from the hinge, produces a slight gap between the valves, too small
for faeces to exit. While the post-anal tuft may be involved in faeces
evacuation, the group of coronate cilia appear slightly separate from
that group (fig. 2). These specialised
cilia also occur in a small tuft below the gill bud (fig
.3), and very occasionally scattered amongst the ventral mantle
cilia. TEM will be used to examine the cells bearing these specialised
cilia.
SEM images will be used to continue the development of cilia 'maps'
-extensions of those found in figure 3
- illustrating cilia location, grouping, size, morphological characteristics
etc. However, SEM images alone can not fully reveal the form and function
of the bivalve larval ciliature. Future investigations of the mantle
will use TEM to provide sectional diagrams for the areas of interest
on the ciliation maps, and confocal laser scanning microscopy for fluorescence
imaging of catecholamines within the larvae, to investigate the nerve
network in relation to the presence, form and function of the mantle
ciliation, expanding the work of Croll et al. (1997).
I would like to thank Dr Simon Cragg and Dr Gordon Watson for guidance
and support with my research, and the Malacological Society of London
for financial support (Centenary Grant 2003) and the opportunity to
present my work to a wider audience through the Molluscan Forums in
2003 and 2004.
References
S. M. Cragg, D.J. Crisp (1991). In Scallops:
Biology, Ecology, and Aquaculture. (Elsevier, New York) pp. 75-132.
R. P. Croll, D. L. Jackson, E. E. Voronezhskaya
(1997). Biological Bulletin 193, 116-124.
T. R. Waller (1981) Smithsonian Contributions
to Zoology, 328: 1-70.
